Brain MRI Report Format: 10 Key Clinical Guidelines & Example
In the field of medical laboratories, the importance of a standardized Brain MRI lab report format cannot be overstated. This format serves as a crucial communication tool, facilitating clear and concise documentation of findings in neuroimaging studies.
A standardized format enhances communication among healthcare providers, contributing to streamlined patient care and fostering collaboration between radiologists, neurologists, and other specialists. Consequently, a meticulously crafted Brain MRI lab report format is instrumental in delivering efficient and effective healthcare services.
10 Key Brain MRI Test Report Format Clinical Guidelines
Below are the 10 key clinical guidelines for formatting a Brain MRI Test report in your pathology laboratory.
1. Patient Information:
- Full name, age, gender, contact details, and unique patient identifier.
- Include relevant medical history for comprehensive understanding.
- Emergency contact information for immediate communication.
- Gather and record any specific patient preferences or considerations.
- Ensure accuracy in transcription of demographic details.
2. Reference Doctor Information:
- Referring physician's full name, contact details, and medical license number.
- Note the referral date and specify the referring physician's specialty.
- Detailed information on the referring institution or clinic.
- Ensure precision in recording the referring physician's instructions.
- Confirm and document any specific preferences indicated by the referring doctor.
3. Body Part Information:
- Specify the body part examined (e.g., Brain) and the positioning (e.g., Supine).
- Mention if contrasting agents were used, slice thickness, and relevant anatomical landmarks.
- Document any specific patient positioning instructions provided by the referring physician.
- Provide clarity on any variations from standard protocols.
- Include information on patient preparation if applicable.
4. Technical Information:
- Provide details about the MRI machine type, model, and magnetic field strength.
- Include information on the imaging protocol used, acquisition time, and reconstruction techniques.
- Document any deviations from the standard imaging procedures.
- Specify any adjustments made during the scan for optimal image quality.
- Ensure that the technical details are consistent and complete.
5. Clinical Indications:
- Offer a detailed clinical history, including the referring doctor's diagnostic questions.
- Consider any relevant medical procedures and present symptoms along with pertinent laboratory results.
- Document patient-reported symptoms accurately.
- Specify any known contraindications or challenges faced during the clinical assessment.
- Include information on the urgency of the examination based on clinical indications.
6. Image Findings:
- Systematically describe brain structures and note any abnormalities detected.
- Include details on the location, size, and characteristics of lesions.
- Compare findings with previous imaging, if available, and identify variations from normal anatomy.
- Document any incidental findings observed during the examination.
- Ensure consistency in reporting abnormal findings across all relevant sections.
7. Impressions:
- Provide a summarized diagnosis or assessment.
- Offer considerations for differential diagnoses and recommend follow-up or additional tests.
- Suggest clinical correlations and highlight prognostic indicators.
- Specify the degree of certainty or confidence in the provided impressions.
- Clearly communicate the clinical significance of the findings for the referring physician.
8. Dr Signature and Date:
- Ensure a clear and legible signature with the date of report generation.
- Include credentials, medical license number, and comply with legal requirements.
- If applicable, use a digital signature for added authenticity.
- Ensure consistency in the signature across all reports.
- Confirm the accuracy of the date and time stamp on the report.
9. QR Code Authenticity and Barcode:
- Implement a unique QR code for report authentication.
- Use a scannable barcode for efficient record-keeping.
- Link to the patient's electronic health records (EHR) for quick access.
- Ensure data integrity and security through these codes.
- Regularly update and validate the QR code for accuracy.
10. Diagnostic Laboratory Details:
- Include the laboratory name, logo, and contact information.
- Specify accreditation and certification details.
- Provide the report issuance date and ensure compliance with regulatory standards.
- Clearly communicate any specific instructions for result interpretation.
- Include information on any affiliated diagnostic networks or collaborative partnerships.
Also Check
Drlogy Plus For Complete Digital Solutions for Doctors, Clinics, Hospitals & Labs to Enhance Patient Experience
Brain MRI Report Format Sample
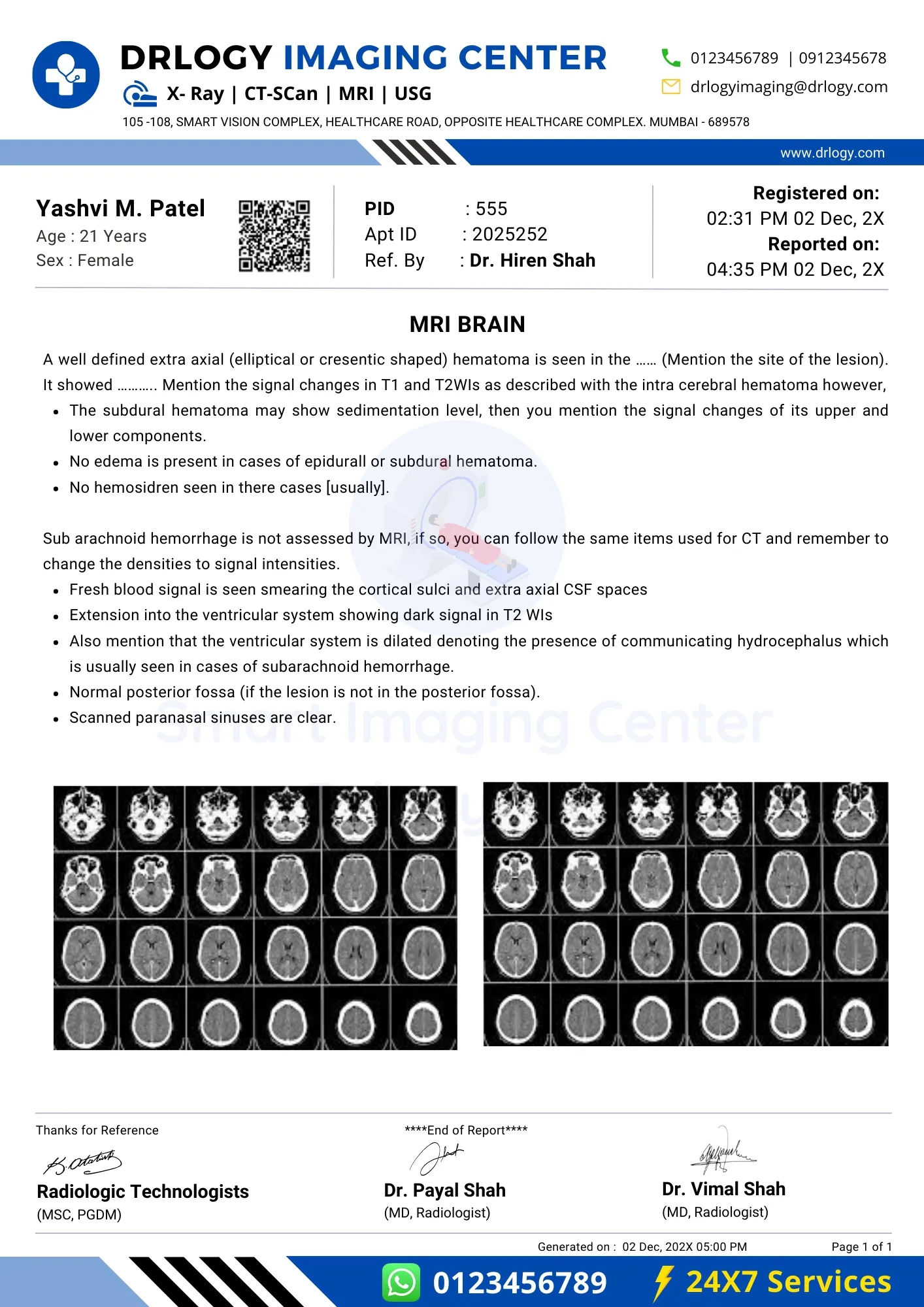
Brain MRI Report Format
Here is a Brain MRI report PDF format, highlighting its significance in the pathology laboratory.
Drlogy Pathology lab software plays a pivotal role in ensuring a Brain MRI Report Format. Additionally, Pathology lab software automates many aspects of the testing process, from sample handling to data analysis. Drlogy Pathology Software provides healthcare providers with real-time access to Brain MRI results, enabling timely decision-making and faster patient care.
- Overall, adhering to these clinical guidelines for Brain MRI report format ensures precision, transparency, and reliability in diagnostic reporting.
- This standardized approach not only enhances communication between healthcare providers but also plays a pivotal role in delivering accurate and timely insights crucial for effective patient care and treatment planning.
- Drlogy Plus For Complete Digital Solutions for Doctors, Clinics, Hospitals & Labs to Enhance Patient Experience.
Reference
- Magnetic resonance imaging of the brain - Wikipedia [1].
- Magnetic Resonance Imaging of the Living Brain - NCBI [2].
- Brain MRI - Drlogy [3].


